Short Communication - American Journal of Physiology, Biochemistry and Pharmacology (2023)
Microbial Indicators within Rotations and Tillage Systems in Grapes Cultivation
Hamid kheyrodin*Hamid kheyrodin, Department of Physiology, Semnan University, Semnan, Italy, Tel: 02331535548, Email: hamid.kheyrodin@semnan.ac.ir
Received: 17-Apr-2023, Manuscript No. AJPBP-23-96217; Editor assigned: 20-Apr-2023, Pre QC No. AJPBP-23-96217 (PQ); Reviewed: 05-May-2023, QC No. AJPBP-23-96217; Revised: 19-Jun-2023, Manuscript No. AJPBP-23-96217 (R); Published: 27-Jun-2023
Abstract
Medicinal properties attributed to the Grape (benefits) Leaves have venotonic, vasoprotective, astringent and diuretic effects. The fruits are vitaminics, tonics, anticancer, hepatoprotective, promote hair growth and prevent ischemic processes. Cover crops are increasingly adopted in viticulture to enhance soil quality and balance the vegetative and reproductive growth of vines. Nevertheless, this sustainable practice has been only recently used for table grape viticulture, with results often contrasting. The aim of this study was to assess the effect of a fescue (Festuca arundinacea Schreb.) cover crop on soil quality, yield and grape qualitative parameters. Soil organic Carbon (C), total Nitrogen (N), Microbial Biomass C (MBC), β-Glucosidase (BGLU) and Alkaline Phosphomonoesterase (APME) activities were assessed during different growing winter season. The trend of soil chemical and microbiological properties was jointly influenced by the soil management system, growing season and phenological stage. Compared to conventional tillage, cover crops increased, on average, soil organic C, total N, MBC, BGLU and APME by 136%, 93%, 112%, 100% and 62%, respectively. Slight or no effects of cover crops were observed on grape quality and yield, except for 2022. This study reveals that cover crops strongly enhance soil quality in the short-term, with potential advantages for grape production in the long term.
Keywords
Vitis vinifera; Soil quality; Soil microbial biomass; β-glucosidase; Alkaline phosphomonoesterase; Grape medicinal properties
Introduction
Grapevine (Vitis vinifera L.) is one of the main fruit crops cultivated all over the world. Agricultural management practices influence soil microbial communities, creating niche environments that favor certain microbes [1,2]. Management practices can include crop rotation, tillage, N fertilization, cover cropping, etc. By selecting management practices or combining them the soil environment is altered, as are essential soil processes. These can include residue decomposition, nutrient and water cycling, aeration and gaseous interactions, development of soil aggregates, Soil Organic Matter (SOM) dynamics, and biodiversity measures [3,4]. Crop rotation is a common management practice with benefits that include pest and disease control and yield improvement and stabilization [5-7]. Tillage is another tool used to improve yields by creating a more favorable environment for cash crop growth. In systems of high organic matter, tillage ensures a clean seedbed for early growth by reducing compaction, improving aeration, increasing soil temperature and removing weed competition [8,9]. Lastly, N fertilization is a common practice used to enhance yields, and that influx of previously scarce N reshapes potential N dynamics controlled by soil microbial communities [10]. Given the benefits of crop rotation, tillage and N fertilization on crop yields, their implementation is widespread, which affects the soil microbial community. Crop rotation and fertilization alter the quantity and quality of crop residues, root exudates, and subsequent rhizodeposits [11-13]. Results from a meta-analysis by Ouyang, et al. showed that crop rotation and soil pH influenced N cycling by changing the Ammonia Oxidizing Bacteria (AOB) and Archaea (AOA) community dynamics, as well as denitrifiers. Crop rotation increased AOB levels compared to monocultures; AOA was unaffected. However, neutrophilic soil conditions led to an increase in both AOA and AOB. Furthermore, N fertilization increased the abundance of AOA, AOB and denitrifies [14]. In a remarkably long term study on the Morrow Plots in Urbana, IL, treatments of crop rotation and N fertilization have been in place since 1876; shifts in microbial functions related to substrate utilization were affected by fertilizer treatments more than crop rotation given the chronic nutrient limitations and changes in soil chemistry [15].
Smith, et al. reported that crop type and tillage altered species composition, but not quantity and quantity and diversity metrics from Indiana corn (Zea mays L.)–soybean (Glycine max (L.) Merr.) rotations. Compared to crop rotation, tillage had a larger effect on nutrient levels, which was a better predictor for microbial community composition [16].
The effects of tillage on the soil microbial community include mechanically disrupting growth and distribution, destroying soil aggregates, reducing soil moisture, increasing soil temperature, and degrading Soil Organic Matter (SOM) [17,18]. In a global meta-analysis looking at the effects of tillage, Zuber and Villamil observed that NT systems have greater microbial biomass and enzymatic activities. Furthermore, de Graaff, et al., also using a meta-analytic approach, showed that tillage decreased bacterial biodiversity, however, it did not affect fungi. Previously when technology was a limiting factor, using broad inference measurements was the best available technique for explaining how the soil microbiome responds to management factors. However, new metagenomic approaches better characterize the microbial community composition and function and its relationship with soil properties and agronomics. Diversity and richness metrics represent the variability within a single sample (α-diversity) and among communities (β-diversity). Using Quantitative Polymerase Chain Reaction (qPCR), functional microbial genes, such as nirK, which is involved in denitrification, are analyzed for treatment effects. Lastly, using primers for each major taxonomic group (bacteria, fungi and archaea, PCR amplification produces a vast pool of amplicons. From that pool of hundreds to thousands of individual amplicons, high throughput sequencing with Illumina yields a deep inventory of Amplicon Sequence Variants (ASVs), from where indicator microbes can be selected and characterized. Indicator microbes usually refer to an ASV that explains variability in a dataset. Studies on indicator microbes have shown that organic matter inputs and pH alter the cycling of N and C, resulting in significant changes in soil biological properties [15]. Given the complexity of using metagenomics to identify indicator microbes, field studies are scarce, especially from a long-term setting. A few long term studies (15–130 years) have determined indicator microbes from typical cropping systems, though none have analyzed crop rotation and tillage simultaneously.
As these are the most common tools used by growers to improve yields, a thorough investigation of these indicator microbes is necessary. We hypothesized that our treatments of continuous corn and soybean would show contrasting effects on microbial taxa, with rotated corn soybean having intermediate results, not different from either monoculture. We also hypothesized that AOB and fungi would have elevated abundances in the continuous corn treatment, with AOA increasing in the continuous soybean treatment. Therefore, the objective of this investigation was to identify microbial taxa that were responsive to crop rotation and tillage from a long-term, stable trial (20+years). The results will add valuable primary information on how the soil microorganisms shift in response to common agricultural management practices.
Materials and Methods
Experimental site description and management practices
The experiment was conducted at the semnan university agricultural research and demonstration center (35.2256° N, 54.4342° E). The study was established in 2022 and a complete description of the site can be found in kheyrodin and Antoun 2911. Briefly, soils were comprised of highly fertile silty clay loam and silt loam soil series (Muscatune 43%, Sable 40% and Osco 17%) [2]. The study was designed in a split-plot arrangement of 4 rotation levels and 2 tillage levels in a randomized complete block design with 4 replications (blocks). The main plots (22 m long by 12 m wide) were crop rotation treatments, which Consisted of Continuous (CCC) (Figure 1).
Were characterized for the following physicochemical properties: Texture [18], pH (both in H2O and in a CaCl2 solution, using in both cases a soil/solution of 1:2.5, m/v), Electrical Conductivity (EC), organic C content (Walkley–Black method), total N content (Kjeldahl method), total and active CaCO3 and available P (Olsen method). All the chemical analyses, except for texture and CaCO3 determination, were carried out following the standard procedures for soil analyses reported by Kheyrodin.
Soil Microbial Biomass C (MBC) was measured through the fumigation-incubation-extraction method. Each field-moist soil sample (containing 50 g oven-dried soil) was divided in half: A non-fumigated half (control) was immediately extracted with 100 mL 0.5 M K2SO4 for 30 min, whereas the remaining part was fumigated with chloroform in the dark, for 24 h at 25°C. After fumigation, CHCl3 was removed and soil samples were extracted as indicated for the control. Organic C in the soil extracts was measured using the Springer–Klee method.
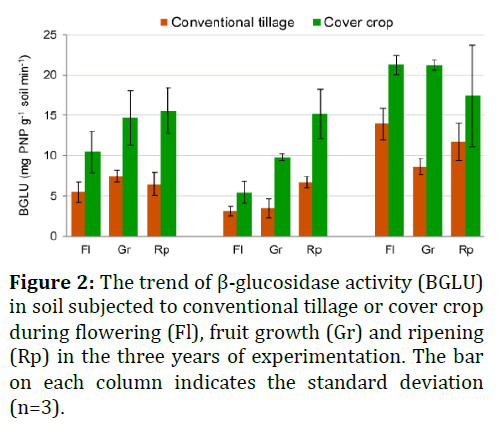
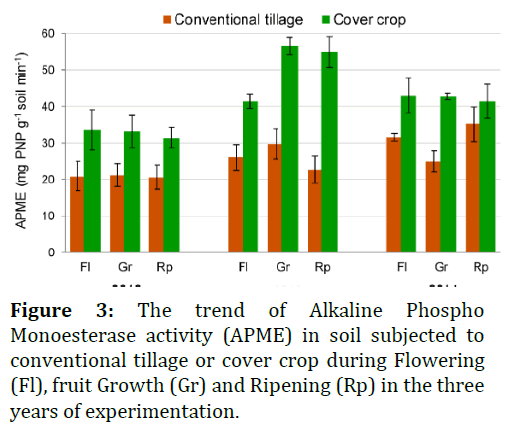
The β-Glucosidase (BGLU) activity of soils was determined according to Kheyrodin and Antoun [17], measuring spectrophotometrically (at 400 nm wavelength) the amount of p-nitrophenol (PNP) released after 1 h of soil incubation with a p-nitrophenol-β-D glucoside solution, at 37°C. TheAlkaline Phospho Monoesterase (APME) activitywas determined following the proceduredescribed by kheyrodin and antoun [1,17]. Briefly,soil was added with a buffered (pH 11) p-nitrophenyl phosphate solution and after 1 h ofincubation at 37°C, the amount of PNP released asa result of the hydrolytic activity was measuredspectrophotometrically at 400 nm wavelength.
Results and Discussion
The result show in Figures 2 and 3. The great abundance and diversity of microorganisms in soil have high metabolic potentials. Since microorganisms microorganismsare generally growth limited in soils, they may poorly exploit their capabilities. The present study demonstrates that the fescue cover crop strongly improves, in the short term (three years), the soil chemical and microbiological properties of a table grape vineyard. In particular, the average increase of 136% of organic C in the cover crop treatment is impressive, considering the very low initial organic C content of the soil (2.6 g kg-1). This enhanced C sequestration, along with the increased total N content and root biomass (due to the absence of tillage passes), stimulates the soil microbial growth.
Conclusion
In this review too we conducted that grapes are a rich source of bioactive molecules including phenolic acids, flavonoids, anthocyanins, stilbenes and lipids. These are the compounds which contribute to the health benefits of grape and grape-derived products. They possess antioxidant, antimicrobial, anti-inflammatory, and anti-carcinogenic activities and have wide applications in food and nutraceutical industries. Use of grape extracts rich in these bioactive compounds are linked to reduced incidence of cardiovascular disease and its major risk factors including hypertension (high blood pressure); a clinical condition associated with high mortality worldwide. Therefore, considerable attention has been given to grape-based products to alleviate and treat hypertension.
Acknowledgments
Authors thank Mohamad Rahimi for the technical support given during the experimental work.
References
- Kheyrodin H, Antoun H. Tillage and manure effect on soil physical and chemical properties and on carbon and nitrogen mineralization potentials. Afr J Biotechnol 2011;10(48):9824-30.
- Kheyrodin H, Ghazvinian K, Taherian M. Tillage and manure effect on soil microbial biomass and respiration, and on enzyme activities. Afr J Biotechnol 2012;11(81):14652-659.
- Kheyrodin H. Important of soil quality and soil agriculture indicators. Acad J Agric Res 2014;2(11):231-38.
- Kheyrodin H, Ghazvinian K. DNA purification and isolation of genomic DNA from bacterial species by plasmid purification system. Afr J Agric Res 2012;7(3):433-42.
- Kheyrodin H, Ghazvinian K. Effect of climate change on soil global microorganisms. J Biol Chem Res 2012;29(2):310-19.
- Kheyrodin H. Isolation and identification of new eleven constituents from medicinal plant. Int J Nutr Metab 2009;1:14-9.
- Kheyrodin H. Importance of wheat farming in Iran: An overview. Ann Agric Envi Sci 2016;1(02):40-8.
- Kheyrodin H. Methodology for measurement of enzyme activity in soil. World J Biol Med Sci 2014;1:18-25.
- Keyrodin H, Ghazvinian K. The toxicity material extraction from Euphorbia species. Iran Agric Res 2013;31(2):65-73.
- Kheyrodin H, Ghazvinian K. Assessment of plant extract toxicity in Euphobial spease. J Recent Adv Agric 2012;1(3):77-83.
- Kheyrodin H, Ghazvinian K. Study of some plant inhabiting fungi. J Biol Chem Res 2012;29(2):121-32.
- Kheyrodin H, Antoun H. Measurement of microbial activity and applicability of dissolved DNA as indicator of microbial activity in pasturage soil. Afr J Agr Res. 2011;6(14):3445-50.
- Kheyrodin H, Ghazvinian K. Bioactive and toxic products found in Euphorbia Sp. J Rec Adv Agri 2012;1(4):103-111.
- Kheyrodin H, Antoun H. Effect of tillage and manure application on soil microbial biomass and respiration and on enzymes activities. World Congr Soil Sci 2012;14-21.
- Kheyrodin H. Study of the soil DNA extraction and recovery procedures. J Med Pharm Allied Sci 2017;3(859):859.
- Kheyrodin H, Ghazvinian K. Soil DNA isolation to use in Polymerase Chain Reaction (PCR) amplification. Afr J Agric Res 2015;10(11):1158-63.
- Kheyrodin H, Antoun H. Long-term tillage and manure effect on soil physical and chemical properties and carbon and nitrogen mineralization potentials. Iran Agric Res 2008;25(1.2):1-4.
- Kheyrodin H. The study of PCR and soil DNA variation. Academia J Biotechnol 2013;1(5):62-70.
Copyright: © 2023 The Authors. This is an open access article under the terms of the Creative Commons Attribution Non-Commercial Share A like 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.