Short Communication - American Journal of Physiology, Biochemistry and Pharmacology (2022)
The Pestilential Transfiguration: Barrett’s Oesophagus
Anubha Bajaj*Anubha Bajaj, Department of Pathology, Panjab University, New Delhi, India, Email: anubha.bajaj@gmail.com
Received: 22-Apr-2022, Manuscript No. AJPBP-22-61587; Editor assigned: 25-Apr-2022, Pre QC No. AJPBP-22-61587 (PQ); Reviewed: 09-May-2022, QC No. AJPBP-22-61587; Revised: 16-May-2022, Manuscript No. AJPBP-22-61587 (R); Published: 24-May-2022
About the Study
Barrett’s oesophagus is a premalignant condition associated with mucosal metaplasia of the lower oesophageal segment from stratified squamous epithelium to simple columnar epithelium with intermingled intestinal goblet cells.
Intestinal metaplasia arising in Barrett’s oesophagus represents progression of metaplasia towards dysplasia and frank adenocarcinoma. Generally, dysplastic lesions in Barrett’s oesophagus are predominantly situated within posterior wall of oesophagus.
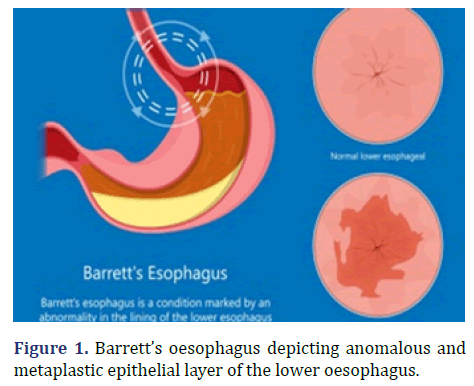
Barrett’s oesophagus was initially described by Philip Rowland Allison in 1946 and subsequently nomenclated by Norman Barrett in 1950 [1,2]. Barrett’s oesophagus is engendered due to chronic exposure to acidic gastric contents secondary to reflux oesophagitis. Possible emergence of Barrett’s oesophagus is enhanced in individuals with central obesity. Barrett’s oesophagus exhibits a male preponderance with a male to female proportion of 10:1. Barrett’s oesophagus depicting anomalous and metaplastic epithelial layer of the lower oesophagus is show in Figure 1.
The condition is associated with possible metamorphoses to oesophageal adenocarcinoma demonstrating a proportionate morality exceeding >85%. Caucasian males beyond >50 years with clinical symptomatology exceeding >5years are associated with possible malignant metamorphosis in Barrett’s oesophagus [2,3].
Majority of individuals incriminated with Barrett’s oesophagus are asymptomatic. Nevertheless, the premalignant condition exhibits manifestations such as frequent, chronic acid reflux, dysphagia, hematemesis, retrosternal pain and odynophagia with consequent cachexia [2,3].
Barrett’s oesophagus occurs on account of chronic inflammation, principally engendered with Gastro-oesophageal Reflux Disease (GERD) wherein the acidic gastric, small intestinal and pancreatic contents along with bile induce cellular degradation within lower oesophageal tract [2,3]. Bulimia may significantly contribute to emergence of Barrett’s oesophagus on account of severe acid reflux.
Bile acids accumulating within the oesophagus as a consequence of acid reflux may initiate carcinogenesis. Besides, diverse mucosal genetic anomalies are associated with possible emergence of oesophageal adenocarcinoma [2,3]. Nevertheless, the condition may be devoid of symptomatic acid reflux. Characteristically, Barrett’s oesophagus exhibits metaplastic columnar epithelium replacing the normal stratified squamous epithelial layer of lower oesophageal tract [2,3]. Metaplastic, secretory columnar epithelium coating the lower oesophagus confers the probability of emerging oesophageal adenocarcinoma [2,3].
Cogent detection of epithelial goblet cells or intestinal metaplasia is a pre-requisite for categorizing Barrett’s oesophagus. Divergent metaplastic columnar cells may be admixed with foci of intestinal or colonic metaplasia [2,3]. Besides, appropriate distinction between goblet cells of submucosal gland ducts and true foci of Barrett’s oesophagus comprised of specialized columnar metaplasia is necessitated [2,3].
Cellular component of Barrett’s oesophagus is classified as
• Non Dysplastic Epithelium
• Low Grade Epithelial Dysplasia
• High Grade Epithelial Dysplasia
• Overt Carcinoma
Histochemical, Alcian blue stain with a pH of 2.5 can be employed to demarcate secretion of true intestinal- type mucins from various mucinous secretions of gastrointestinal tract [2,3].
Contemporary immunohistochemistry with antibodies to CDX-2, a molecule which is specific for midgut and hindgut, intestinal cellular derivatives can be adopted to discern true intestinal-type metaplastic epithelial cells [3,4]. Anterior Gradient Protein 2 (AGR2) homolog is a biomarker which is elevated in Barrett’s oesophagus and can segregate Barrett epithelium from normal oesophageal epithelium [3,4].
Additionally, immune reactivity to p53, Her2 and p16 signifies the expression of diverse genetic pathways with probable progression to dysplasia. Intestinal metaplastic cells can be immune reactive to Cytokeratin 7 (CK7) and immune nonreactive to Cytokeratin (CK20) [3,4]. Barrett’s oesophagus is appropriately discerned with endoscopy wherein characteristic morphology of lower oesophagus can be subjected to direct inspection [3,4].
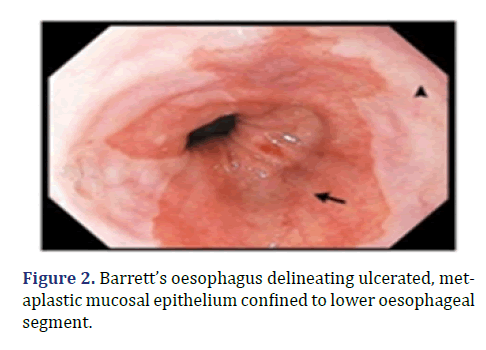
Endoscopic screening is recommended in male subjects beyond >60 years with symptoms of chronic acid reflux unresponsive to medical therapy. Foci of intestinal or colonic metaplasia can be macroscopically identified with a gastroscope [2,4]. Barrett’s oesophagus delineating ulcerated, metaplastic mucosal epithelium confined to lower oesophageal segment is shown in Figure 2.
Tissue samples can be obtained with screening endoscopy at one centimeter to two centimeters from the gastroesophageal junction. Cogent tissue sampling of incriminated lower oesophagus is optimal for detecting and categorizing Barrett’s oesophagus [2,3].
Additionally, a cyto-sponge which can be swallowed may be employed to obtain pertinent cellular samples. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) such as aspirin may circumvent emergence of oesophageal carcinoma in Barrett’s oesophagus [2,4]. Anti-reflux surgical procedures remain ineffective in circumventing emergence of oesophageal carcinoma [2,4].
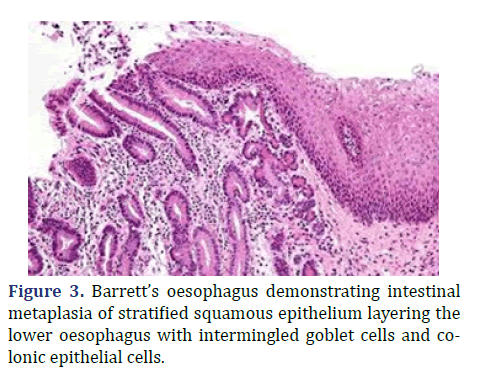
Lesions layered with non-dysplastic epithelium or exhibiting low grade epithelial dysplasia can be appropriately managed with annual endoscopic observation or radiofrequency ablation. Contemporary modality of balloon-based radiofrequency ablation is efficacious in treating Barrett’s esophagus and dysplasia [2,4]. High grade dysplasia and antecedent adenocarcinoma can be subjected to endoscopic resection or radiofrequency ablation. Advanced oesophageal adenocarcinoma can be appropriately alleviated with surgical resection or palliation [2,4]. Barrett’s oesophagus demonstrating intestinal metaplasia of stratified squamous epithelium layering the lower oesophagus with intermingled goblet cells and colonic epithelial cells is shown in Figure 3.
High grade dysplasia can be treated with surgical eradication of oesophagus or oesophagectomy or endoscopic manoeuvers as endoscopic mucosal resection or mucosal ablation [3,4]. Laser therapy can be employed for alleviating severe epithelial dysplasia. Proton-pump inhibitors appear competent in restricting progression of oesophageal adenocarcinoma [3,4]. Photodynamic therapy with photofrin appears efficacious in eliminating foci of epithelial dysplasia, in contrast to singular employment of proton- pump inhibitors [3,4].
Conclusion
Overt oesophageal adenocarcinoma can be managed with surgical intervention, radiation therapy or systemic chemotherapy. Nissen’s fundoplication is an operative manoeuver which can decimate gastric acid reflux into the oesophagus. Annual surveillance can be adopted in order to detect mucosal alterations with possible emergence of epithelial dysplasia, intraepithelial neoplasia and frank adenocarcinoma.
REFERENCES
- Barrett NR. Chronic peptic ulcerz of the œophagus and ‘œsophagitis’. Br J Surg 1950; 38:175-82.
[Crossref] [Google scholar] [pubmed]
- Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2022;117:27-56.
[Crossref] [Google scholar] [pubmed]
- Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol 2021;18:432-43.
[Crossref] [Google scholar] [pubmed]
- Shaheen NJ, Falk GW, Iyer PG, Souza RF, Yadlapati RH, Sauer BG, et al. Diagnosis and management of barrett's esophagus: An updated ACG guideline. Am J Gastroenterol 2022;117:559-87.
[Crossref] [Google scholar] [pubmed]
Copyright: © 2022 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.